Atoms and chemical species lose or gain electrons when they react in order to gain stability. When electrons gain or lose energy they jump between shells as they are rotating around the nucleus.
Electronic Configuration Of An Atom Electronic Configuration Of
When a molecule is oxidized it loses energy.

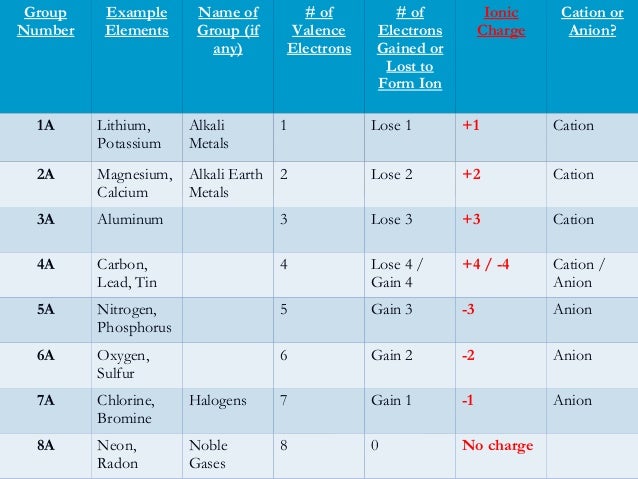
N gain or lose electrons. Loss of electrons leaves an atom with a net positive charge and the atom is called a cation. Oxygen has 6 electrons in it valence second shell and can lose 6 electrons or gain 2 electrons to make its valence shell full and stable. Theres more than just those two things to consider but that answers the question most of the time.
In contrast when a molecule is reduced it gains one or more electrons. Atoms that gain electrons would have a negative charge and are called anions. The number of electrons depends on their position on the Periodic table in simple terms.
The outermost shell of the sodium ion is the second electron shell which has eight electrons in it. And even though many teachers fall into this trap be careful with saying want. 3302020 When atoms lose or gain electrons they become what are called ions.
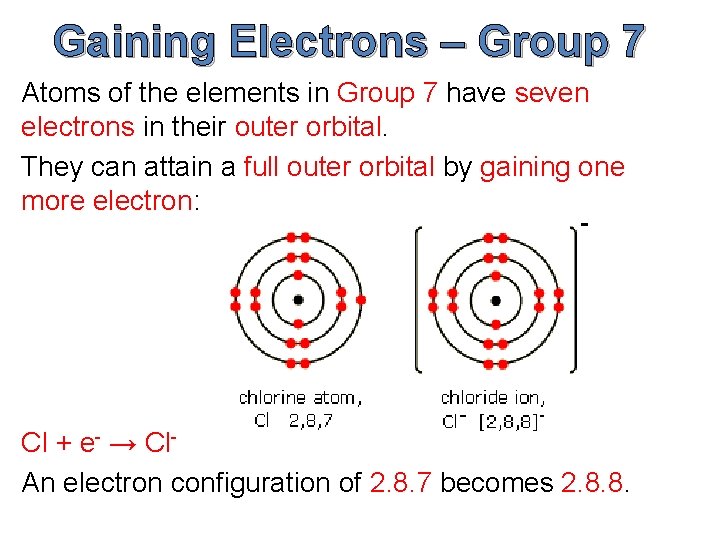
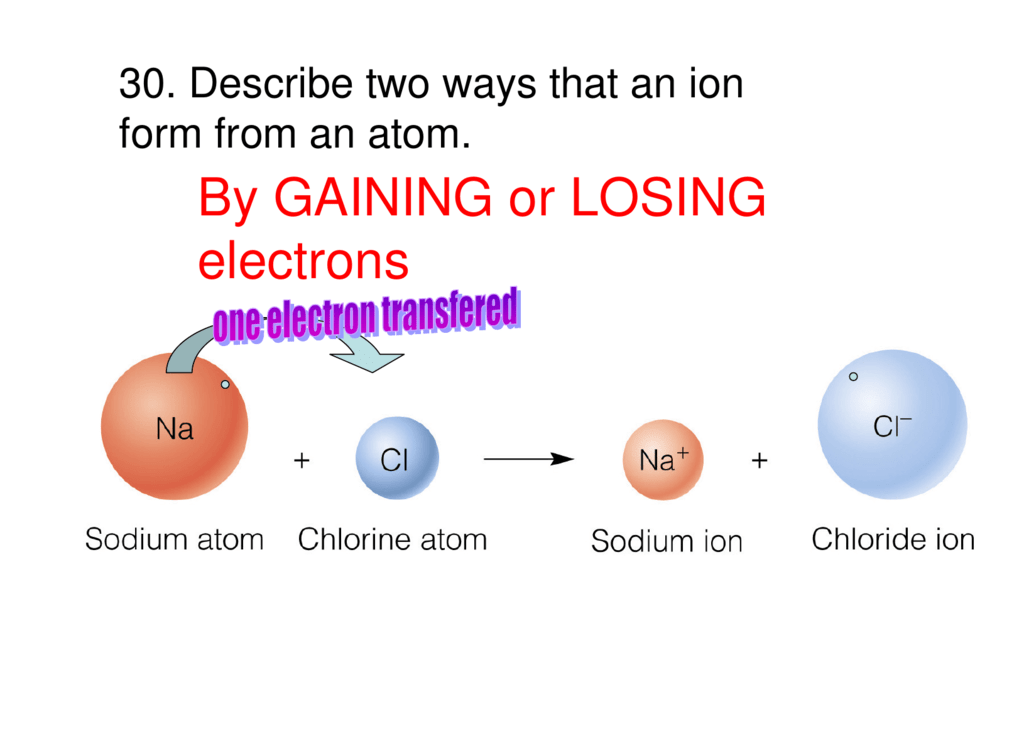
Negative Ion - Occurs when an atom gains an electron negative charge it will have more electrons than protons. Thus typically metals with nearly empty outer shells lose electrons to non-metals thereby forming positive ions. The following image shows Na losing an electron and Cl gaining an electron Thus the Na becomes Na.
Since the number of protons and electrons is not equal after electrons have been gained or lost there is a net charge. Metalloids and some metals can be can lose or gain electrons. As you might have guessed the molecule gains energy in the process.
CeNa rightarrow Na e- The cation produced in this way Na is called the sodium ion to distinguish it from the element. They achieve the same number of valence electrons as the Noble Gases in the last column Gain or Loss of Electrons The positive and negative charges are not balanced. 6152015 Many atoms gainlose electrons with the hope of having the same number of electrons as the closest noble gas in the periodic table.
The oxide ion will have a charge of 2 as a result of gaining two electrons. When atoms gain or lose electrons. Types of Bonds It is the electric forces between oppositely.
When an ionic compound forms the. Therefore Potassium wants to lose one electron. What is the formula of.
Therefore in order for these atoms to bond they either lose or gain their electrons. The outermost shell of the sodium ion is the second electron shell which has eight electrons in it. This is not always true as elements such as nitrogen can lose electrons to become positive.
In terms of gaining losing electrons. Positive Ion - Occurs when an atom loses an electron negative charge it has more protons than electrons. 962018 Oxidation occurs when a molecule loses an electron or increases its oxidation state.
And Na 1 10 electrons 11 protons. When an ionic compound forms the more electronegative element will gain electrons and the less electronegative element will lose electrons. 11252018 A neutral sodium atom is likely to achieve an octet in its outermost shell by losing its one valence electron.
Hydrogen is an exception as it will usually lose its electron. Sodium Na with atomic no. Oxygen has an electron arrangement of 2 6 and needs to gain two electrons to fill the n2 energy level and achieve an octet of electrons in the outermost shell.
6222020 Does nitrogen gain or lose electrons in chemical changes. Then as they lose energy by emitting photons they might move back to the second energy level shell or even to the first energy level shell. Gain of electrons leaves an atom with a net negative charge and the atom is called an anion.
If an atom is near to one that is more interested in electrons then it will lose one. F 1 10 electrons 9 protons. 452016 A neutral sodium atom is likely to achieve an octet in its outermost shell by losing its one valence electron.
NAtoms gain or lose electrons to become more stable. 11 by losing one electron from its outermost shell it will attain the nearby noble gas. For example as electrons gain energy from photons small bundles of energy they might move from the second to the third energy level shell.
Depending upon its nearest noble gas electronic configuration the element will lose or gain the electrons to attain the stability. Carbon-12 P 6 N 6 Boron One less proton One more proton One less neutron One more neutron Isotopes. However gain or lose or share depends on what partners are on offer.
If it is next to one less interested then it will gain one. The book uses Potassium as an example. Nickel has 2 electrons in its valence fourth shell and can lose 2 electrons or gain 6 more electrons to make it stable.
Every atom has an infinite set of possible orbital in which electrons can be added. As a genera l rule atoms with fewer than four electrons in their outer shells behave like losers. CeNa rightarrow Na e- The cation produced in this way Na is called the sodium ion to distinguish it from the element.
Atoms that lose electrons will have a positive charge and are called cations. Anion - If we lose or gain electrons we will have a charged atom called an ion. Looking at my table the closest noble gas to Potassium is Argon which has 18 electrons.
812017 In general metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. This is not always true as elements such as nitrogen can lose electrons to become positive. 12122018 It would have to gain six electrons to fill up the valence shell or lose two to vacate the shell.

Ppt Oxidation An Atom Loses One Or More Electrons Powerpoint Presentation Id 5311513

Do Now 1 7 10 Explain In Complete Sentences Why Atoms Are Neutral In Charge Hint Think About The Charges And Numbers Of The Subatomic Particles Reminder Ppt Download

Chapter 8 Chemical Bonding Section 1 Electrons A Chemical Bond Is The Joining Of Atoms To Form New Substances With New Properties Compounds Will Not Ppt Download

What Is The Best Way Of Knowing If An Atom Will Gain Or Lose An Electron Quora

Atomic Electron Shells Animated Presentation Youtube Nursing School Notes Homeschool Science Teaching Science

What Is An Ion And How Do You Make One Ppt Download
By Gaining Or Losing Electrons

Posting Komentar
Posting Komentar